Q.1) MCQ
1.) Which of the following is the product of reaction between methane and chlorine in presence of sunlight ?
a) HCl
b) H2SO4
c) methane
d) ethane
2.)
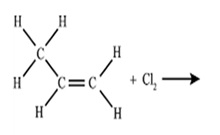
Which of the following is the IUPAC name of product for above reaction?
a.) 1,3-Dichloropropane
b.) 1,2-Dichloroethane
c.) 1,2-Dichloropropane
d.) Chloropropane
3.)

Which hydrocarbon is the reactant here?
a) Propene
b) Ethene
c) Butene
d) Methane
4.) PVC is ——————–
a) Polyvinyl carbon
b) Polyvinyl chloride
c) Polymeric carbon
d) Polythene chloride
5.) The polymer of isoprene is ——————-
a) PVC
b) Natural rubber
c) Teflon
d) Polythene
6.) Hydrocarbon burn to produce CO2 and H2O with heat and light, the process is called
a) Inflation
b) Combustion
c) Reduction
d) Thermal cracking
7.) Which of the following is wood spirit?
a) Ethanol
b) Carbon
c) Hydrocarbons
d) Methanol
8.) Which of the following is used as a fuel?
a) Methanol
b) Propanol
c) Ethanol
d) Butanol
9.) IUPAC name of the following compound?
CH3-CH2-COOH
a) Ethanoic acid
b) formic acid
c) Butanoic acid
d) Propanoic acid
10.) Which of the following is the product of reaction between alcohols and carboxylic acid?
a) Esters
b) Acids
c) Formic acid
d) Ethanoic acid
Q.2) VERY SHORT ANSWERS.
1.) Define soaps and detergents ?
2.) What is thermal cracking?
3.) What is combustion?
4.) define polymerisation.
5.) define substitution reaction.
6.) what is addition reaction
Q.3) SHORT ANSWER QUESTIONS.
1.) Give the reaction of polymerisation of Ethene ?
2.) give the example of each?
3.) Give the reaction of ethanoic acid and ethanol in presence of conc. Sulphuric acid. Give the IUPAC name of the product.
4.) How ethanoic acid is prepared In industries?
5.) Give the structural formula of methanoicacid , ethyl ethanoate , methanol , propanoic acid ?
Q.4) LONG ANSWER QUESTIONS.
1.) Complete the table
NO. | COMPOUND | IUPAC name |
| 1. | CH3-CH2-CH2-COOH | |
| 2. | CH3-CH2-COO-CH3 | |
| 3. | CH3-COOH | |
| 4. | CH3-CH2-CH2-CH2-OH | |
| 5. | CH3-CH2-CH2-CH2-CH3 |
2.) Match the following columns A,B,C and rewrite the table.
| Reactants (A) | Products(B) | Name of the reaction (C) |
| CH3-CH3 + Cl2 | CO2 + H2O | Addition reaction |
| C2H6 + O2 | CH2=CH2 + CH4 | Thermal cracking |
| CH2=CH2 + H2 | CH3-CH3 | Substitution reaction |
| CH3-CH2-CH3 | CH3-CH2-Cl + HCl | Combustion |
Answer Sheet
Chemical Reactions of Organic Compounds
Q.1) MCQ
a) HCl
c) 1,2-Dichloropropane
a) propene
b) polyvinyl chloride
b) natural rubber
b) combustion
d) methanol
c) ethanol
d) propanoic acid
a) esters
Q.2) VERY SHORT ANSWERS.
1.) Soaps are the salts formed when oils and fats reacts with alkalies. They are esters of fatty acids and glycerol.
Detergents are cleansing agents made from hydrocarbons obtain from coal and petroleum.
2.) Some hydrocarbons with high molecular masses, when heated in absence of air undergo decomposition to form hydrocarbons with lower molecular masses , this process is called thermal cracking.
3.) When hydrocarbons burn they combine with the oxygen in the air to form CO2 and H2O along with heat and light. This process is called combustion.
4.) Polymerisation is the process in which a large number of simple molecules combine under suitable conditions to form complex molecule.
5.) A reaction in which an atom or a group in a compound is replaced by another atom or a group is called substitution reaction.
6.) Reactions in which unsaturated organic compounds with double bond or triple bonds react with other molecules to form saturated compounds are called addition reactions.
Q.3) SHORT ANSWER QUESTIONS.

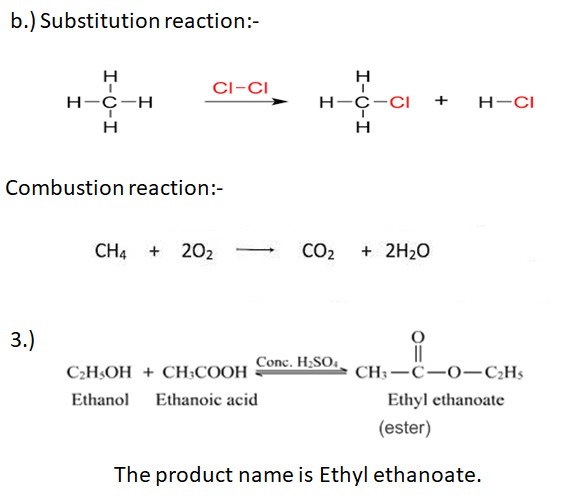
4.) Ethanoic acid can be manufactured by treating methanol with carbon monoxide in presence of catalyst.
CH3-OH + CO → CH3-COOH
5.)
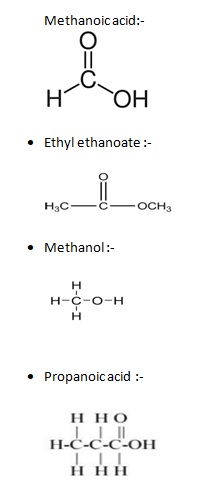
Q.4.) LONG ANSWER QUESTIONS.
1.) Complete the table
| COMPOUND | ||
| 1. | CH3-CH2-CH2-COOH | Butanoic acid |
| 2. | CH3-CH2-COO-CH3 | Methyl propanoate |
| 3. | CH3-COOH | Ethanoic acid |
| 4. | CH3-CH2-CH2-CH2-OH | Butanoic acid |
| 5. | CH3-CH2-CH2-CH2-CH3 | Pentane |
| Reactants (A) | Products(B) | |
| C2H6 + O2 | CO2 + H2O | Combustion reaction |
| CH2=CH2 + H2 | CH3-CH3 | Addition reaction |
| CH3-CH2-CH3 | CH2=CH2 + CH4 | Thermal cracking |



